Urgent alert: TGA issues warning about potentially harmful painkillers in pharmacies
In a recent development that has left both consumers and health professionals concerned, the Therapeutic Goods Administration (TGA) has issued a safety alert for a popular painkiller that was never intended for direct sale to the public.
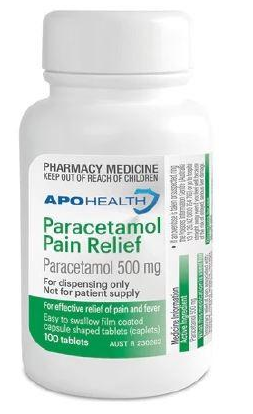
The product in question, Apohealth Paracetamol Pain Relief 500mg film-coated tablets, has been found on pharmacy shelves and online stores, raising serious safety concerns.
The TGA, Australia's regulatory authority for therapeutic goods, has warned that these tablets, sold in a bottle with a green and white label, lacked child-resistant packaging.
This is a significant oversight, as paracetamol, while a common over-the-counter medication, can be dangerous if consumed in large quantities, especially by children.
Paracetamol overdose can lead to liver damage and, in severe cases, can be fatal. The maximum recommended dose for adults is 4,000 milligrams per day.
For children, the dose varies based on weight and age. It's crucial to read the labels on all medications, as many contain paracetamol, and unintentional overdoses can occur when multiple paracetamol-containing products are taken simultaneously.
Symptoms of paracetamol overdose can include nausea, vomiting, loss of appetite, and abdominal pain.
If you have purchased this product, it is recommended that you check your medicine cabinet and ensure it is kept out of reach of children.
The label on the bottle also clearly states, 'For dispensing only. Not for patient supply.'
This indicates that the product was intended to be dispensed by a pharmacist, not sold directly to consumers.
The TGA's safety alert noted, 'We are aware that this product may have been sold to some consumers—either through physical pharmacies or online stores.'
If you have concerns about the bottle's safety, it should be returned to the place of purchase.
It's important to note that a separate version of the product, labelled Apohealth Paracetamol Pain Relief 500mg film-coated tablets in blister packaging, is the one intended for direct supply and is not affected by this safety alert.
In case of suspected overdose or unintended consumption of medicine by a child, immediate contact should be made with the Poisons Information Centre on 131 126.

We encourage our readers to share this information with friends and family to ensure the safety of our community.
If you have any concerns or questions about medication safety, don't hesitate to contact your pharmacist or healthcare provider.
Have you or anyone you know purchased this product? Let us know in the comments below.
The product in question, Apohealth Paracetamol Pain Relief 500mg film-coated tablets, has been found on pharmacy shelves and online stores, raising serious safety concerns.
The TGA, Australia's regulatory authority for therapeutic goods, has warned that these tablets, sold in a bottle with a green and white label, lacked child-resistant packaging.
This is a significant oversight, as paracetamol, while a common over-the-counter medication, can be dangerous if consumed in large quantities, especially by children.
Paracetamol overdose can lead to liver damage and, in severe cases, can be fatal. The maximum recommended dose for adults is 4,000 milligrams per day.
For children, the dose varies based on weight and age. It's crucial to read the labels on all medications, as many contain paracetamol, and unintentional overdoses can occur when multiple paracetamol-containing products are taken simultaneously.
Symptoms of paracetamol overdose can include nausea, vomiting, loss of appetite, and abdominal pain.
If you have purchased this product, it is recommended that you check your medicine cabinet and ensure it is kept out of reach of children.
The label on the bottle also clearly states, 'For dispensing only. Not for patient supply.'
This indicates that the product was intended to be dispensed by a pharmacist, not sold directly to consumers.
The TGA's safety alert noted, 'We are aware that this product may have been sold to some consumers—either through physical pharmacies or online stores.'
If you have concerns about the bottle's safety, it should be returned to the place of purchase.
It's important to note that a separate version of the product, labelled Apohealth Paracetamol Pain Relief 500mg film-coated tablets in blister packaging, is the one intended for direct supply and is not affected by this safety alert.
In case of suspected overdose or unintended consumption of medicine by a child, immediate contact should be made with the Poisons Information Centre on 131 126.
Key Takeaways
- A safety alert has been issued for Apohealth Paracetamol Pain Relief 500mg film-coated tablets, which were never supposed to be directly available to consumers.
- These tablets were found on pharmacy shelves, despite their bottles stating: 'For dispensing only. Not for patient supply,' and lack of child-resistant packaging.
- The Therapeutic Goods Administration warned of a potential risk for an accidental overdose if a child can access the medicine in its original container.
- It is recommended consumers who bought the product return it if they are worried about the bottle's safety. Meanwhile, the blister packaging version of the product is not affected by the alert and is safe for direct supply.
We encourage our readers to share this information with friends and family to ensure the safety of our community.
If you have any concerns or questions about medication safety, don't hesitate to contact your pharmacist or healthcare provider.
Have you or anyone you know purchased this product? Let us know in the comments below.