Breakthrough Prostate Cancer Treatment Discovered: Is This The Miracle Patients Have Been Waiting For?
By
- Replies 5
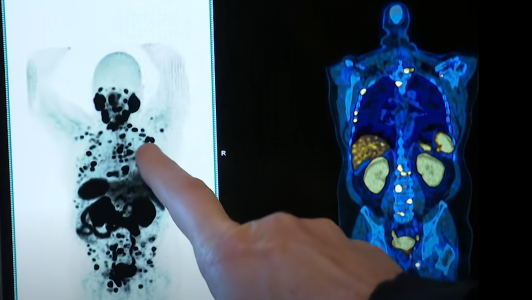
The battle against prostate cancer, a disease that affects a significant number of Australian men, particularly those over the age of 60, has been long and arduous. However, a ray of hope has emerged from the corridors of Sydney's St Vincent's Hospital, where a groundbreaking drug trial is offering new hope to patients with advanced prostate cancer.
The trial, which is a beacon of light for many, has already changed the life of 71-year-old yachtsman Rob Lewis. Diagnosed with metastatic prostate cancer, a condition where the cancer has spread to other parts of the body, Lewis was facing a grim prognosis. The pain that he initially attributed to a racing injury turned out to be a life-altering diagnosis. But thanks to this innovative drug trial, Lewis has experienced a remarkable turnaround. After just three months of treatment, his cancer became 'undetectable,' a term that brings immense relief and joy to anyone familiar with the disease.
The trial, led by Professor Louise Emmett and funded by Movember and the federal government, involved 162 men who were battling advanced stages of prostate cancer. The participants were divided into two groups: one received the drug enzalutamide alone, while the other was treated with a combination of enzalutamide and a novel radiation treatment known as Lutetium-PSMA-617.
The results have been nothing short of extraordinary. Patients on the combination therapy showed an improved response to the treatment and, importantly, experienced fewer side effects. This is a significant development, as the quality of life during cancer treatment is a critical concern for patients and their families. Dr. Megan Crumbaker, an oncologist involved in the trial, highlighted the dual benefits of the treatment, noting its effectiveness and the minimal side effects, which she described as 'the whole shebang.'
The interim findings of this trial have been published in The Lancet Oncology, and they paint a picture of a potential future where prostate cancer could be transformed from a lethal disease with severe symptoms to a chronic condition that patients can manage while continuing to enjoy their lives.
However, there's a catch. The treatment, particularly the Lutetium-PSMA-617 component, comes with a hefty price tag. Currently, there is no funding for this treatment in Australia, which means it's out of reach for many who could benefit from it. Both Emmett and Lewis are advocating for change, hoping to see Lutetium-PSMA-617 become more accessible to patients across the country. 'It should be available to everybody. It's just a game changer,' Lewis passionately stated.
For Lewis, the drug trial has given him a new lease on life. The appreciation for the simple things, the ability to spend precious time with loved ones, and the chance to look after his granddaughter a few days a week are gifts that he attributes to the success of the treatment.
This trial is a testament to the power of medical research and the importance of funding such initiatives. It also serves as a reminder to our readers that participation in clinical trials can sometimes offer access to cutting-edge treatments that are not yet widely available.
As we continue to follow the progress of this trial and the push for funding, we invite our readers to share their thoughts and experiences. Have you or a loved one been affected by prostate cancer? What are your hopes for the future of treatment? Your stories are important, and by sharing them, we can foster a community of support and information.
For those interested in learning more about this trial or seeking support for prostate cancer, there are resources available, such as Prostate Cancer Foundation of Australia (PCFA), which offers guidance and community support for those affected by the disease.
 The journey to conquer prostate cancer is far from over, but with each scientific advancement, we move closer to a world where this disease no longer poses the threat it once did. For now, we celebrate the successes and continue to support the tireless efforts of researchers, clinicians, and patients who are on the front lines of this fight.
The journey to conquer prostate cancer is far from over, but with each scientific advancement, we move closer to a world where this disease no longer poses the threat it once did. For now, we celebrate the successes and continue to support the tireless efforts of researchers, clinicians, and patients who are on the front lines of this fight.
The trial, which is a beacon of light for many, has already changed the life of 71-year-old yachtsman Rob Lewis. Diagnosed with metastatic prostate cancer, a condition where the cancer has spread to other parts of the body, Lewis was facing a grim prognosis. The pain that he initially attributed to a racing injury turned out to be a life-altering diagnosis. But thanks to this innovative drug trial, Lewis has experienced a remarkable turnaround. After just three months of treatment, his cancer became 'undetectable,' a term that brings immense relief and joy to anyone familiar with the disease.
The trial, led by Professor Louise Emmett and funded by Movember and the federal government, involved 162 men who were battling advanced stages of prostate cancer. The participants were divided into two groups: one received the drug enzalutamide alone, while the other was treated with a combination of enzalutamide and a novel radiation treatment known as Lutetium-PSMA-617.
The results have been nothing short of extraordinary. Patients on the combination therapy showed an improved response to the treatment and, importantly, experienced fewer side effects. This is a significant development, as the quality of life during cancer treatment is a critical concern for patients and their families. Dr. Megan Crumbaker, an oncologist involved in the trial, highlighted the dual benefits of the treatment, noting its effectiveness and the minimal side effects, which she described as 'the whole shebang.'
The interim findings of this trial have been published in The Lancet Oncology, and they paint a picture of a potential future where prostate cancer could be transformed from a lethal disease with severe symptoms to a chronic condition that patients can manage while continuing to enjoy their lives.
However, there's a catch. The treatment, particularly the Lutetium-PSMA-617 component, comes with a hefty price tag. Currently, there is no funding for this treatment in Australia, which means it's out of reach for many who could benefit from it. Both Emmett and Lewis are advocating for change, hoping to see Lutetium-PSMA-617 become more accessible to patients across the country. 'It should be available to everybody. It's just a game changer,' Lewis passionately stated.
For Lewis, the drug trial has given him a new lease on life. The appreciation for the simple things, the ability to spend precious time with loved ones, and the chance to look after his granddaughter a few days a week are gifts that he attributes to the success of the treatment.
This trial is a testament to the power of medical research and the importance of funding such initiatives. It also serves as a reminder to our readers that participation in clinical trials can sometimes offer access to cutting-edge treatments that are not yet widely available.
As we continue to follow the progress of this trial and the push for funding, we invite our readers to share their thoughts and experiences. Have you or a loved one been affected by prostate cancer? What are your hopes for the future of treatment? Your stories are important, and by sharing them, we can foster a community of support and information.
For those interested in learning more about this trial or seeking support for prostate cancer, there are resources available, such as Prostate Cancer Foundation of Australia (PCFA), which offers guidance and community support for those affected by the disease.
Key Takeaways
- A drug trial at St Vincent's Hospital in Sydney is offering new hope to patients with advanced prostate cancer.
- Rob Lewis, a participant in the trial, saw his metastatic prostate cancer become 'undetectable' after just three months with minimal side effects.
- The trial, led by Professor Louise Emmett and funded by Movember and the federal government, combines enzalutamide with radiation treatment Lutetium-PSMA-617.
- Interim findings show that the combination therapy shows improved response and fewer side effects, with hopes that Lutetium-PSMA-617 becomes more widely available and funded in Australia.