Are turmeric supplements hurting your liver? TGA issues warning for over 600 medicines
We’ve all heard about the health benefits of turmeric–from boosting brain power to aiding digestive troubles. This ancient spice loved worldwide for its potent antioxidant compounds has even become a staple in many pantries in Australia.
But did you know that taking certain turmeric supplements could risk your health? The Therapeutic Goods Administration (TGA) has issued a warning, revealing the life-threatening risk of liver injury posed by turmeric’s active ingredient: curcumin.
According to the regulator, there have been 18 reports of related liver injury recorded by June 29, and at least one death caused by a medicinal dosage of curcumin.
‘Available evidence shows that there is a rare risk of liver injury from taking Curcuma longa (turmeric) and/or curcumin in medicinal dosage forms,’ the warning stated.
However, the TGA confirmed that the risk for liver injury doesn’t relate to turmeric consumed in typical quantities in food.
But why have these warnings come out now?
Turmeric (Curcuma longa) is just one spice within the Curcuma genus, which naturally produces curcumin. It is also found in Curcuma aromatica, Curcuma zanthorrhiza and Curcuma zedoaria– all of which are now under investigation by the TGA–in light of reports of liver injury caused by medicinal dosages of medications usually made from turmeric or its active ingredient.
Leading the cause for concern is that over 600 medicines listed in the Australian Register of Therapeutic Goods (ARTG) have been found to contain curcumin, and medicines and herbal supplements containing curcumin compounds are readily available without a prescription in supermarkets, health food shops and pharmacies.
So how can you tell if curcumin has been used in a supplement, medication or herbal product?
Unfortunately, it’s not as simple as looking for ‘turmeric’ in the ingredients list.
Curcumin occurs in other Curcuma species used in medicines and supplements. Just to be safe, should look for Curcuma aromatica, Curcuma zanthorrhiza and Curcuma zedoaria on the packaging.
Last year, the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) reported adverse effects of curcumin supplements, which also included cases of hepatitis.
The report mentioned that curcumin has poor bioavailability, meaning that much of the product is not absorbed into the bloodstream before it leaves the body.
However, some products, like piperine–found in black pepper–can naturally enhance the bioavailability of curcumin.
‘Manufacturers have developed various formulations to increase this bioavailability and thereby enhance the effects of curcumin,’ ANSES said.
Here’s the main tip for folks who think they might be taking a curcumin supplement: ANSES recommends limiting daily intakes of curcumin to below 153mg for a 60kg adult.
So if you’re taking turmeric supplements, look out for the daily dosage on the back of the packaging–and make sure it's in line with their recommendations.
TGA also advised those with a history of liver problems to avoid taking medicines or supplements containing the mentioned Curcuma species and/or curcumin.
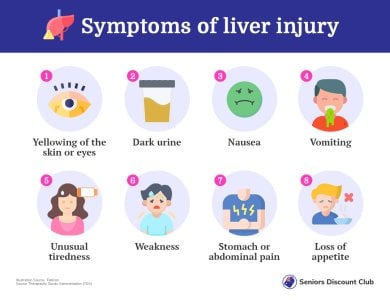
If you or anyone you know is experiencing any of the following symptoms, please stop taking the supplements immediately and seek medical advice:
If you have any questions or concerns about this issue, please discuss it with your healthcare provider.
You may also report suspected side effects for any medicine or supplements with Curcuma species and/or curcumin to TGA here or consult a doctor.
Currently, TGA is monitoring the situation and considering regulatory action, including a warning label consultation. They will publish their recommendations later this year.
‘There is not enough information at this time to conclusively identify which medicines are higher risk,’ TGA said.

The ANSES and the TGA's warning is an important reminder to stay vigilant when taking supplements. Stay safe, folks!
What do you think of this story, dear members? Do you or anyone you know take turmeric as a supplement? Share your thoughts in the comments!
But did you know that taking certain turmeric supplements could risk your health? The Therapeutic Goods Administration (TGA) has issued a warning, revealing the life-threatening risk of liver injury posed by turmeric’s active ingredient: curcumin.
According to the regulator, there have been 18 reports of related liver injury recorded by June 29, and at least one death caused by a medicinal dosage of curcumin.
‘Available evidence shows that there is a rare risk of liver injury from taking Curcuma longa (turmeric) and/or curcumin in medicinal dosage forms,’ the warning stated.
However, the TGA confirmed that the risk for liver injury doesn’t relate to turmeric consumed in typical quantities in food.
But why have these warnings come out now?
Turmeric (Curcuma longa) is just one spice within the Curcuma genus, which naturally produces curcumin. It is also found in Curcuma aromatica, Curcuma zanthorrhiza and Curcuma zedoaria– all of which are now under investigation by the TGA–in light of reports of liver injury caused by medicinal dosages of medications usually made from turmeric or its active ingredient.
So how can you tell if curcumin has been used in a supplement, medication or herbal product?
Unfortunately, it’s not as simple as looking for ‘turmeric’ in the ingredients list.
Curcumin occurs in other Curcuma species used in medicines and supplements. Just to be safe, should look for Curcuma aromatica, Curcuma zanthorrhiza and Curcuma zedoaria on the packaging.
Last year, the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) reported adverse effects of curcumin supplements, which also included cases of hepatitis.
The report mentioned that curcumin has poor bioavailability, meaning that much of the product is not absorbed into the bloodstream before it leaves the body.
However, some products, like piperine–found in black pepper–can naturally enhance the bioavailability of curcumin.
‘Manufacturers have developed various formulations to increase this bioavailability and thereby enhance the effects of curcumin,’ ANSES said.
Here’s the main tip for folks who think they might be taking a curcumin supplement: ANSES recommends limiting daily intakes of curcumin to below 153mg for a 60kg adult.
So if you’re taking turmeric supplements, look out for the daily dosage on the back of the packaging–and make sure it's in line with their recommendations.
TGA also advised those with a history of liver problems to avoid taking medicines or supplements containing the mentioned Curcuma species and/or curcumin.
If you or anyone you know is experiencing any of the following symptoms, please stop taking the supplements immediately and seek medical advice:
If you have any questions or concerns about this issue, please discuss it with your healthcare provider.
You may also report suspected side effects for any medicine or supplements with Curcuma species and/or curcumin to TGA here or consult a doctor.
Currently, TGA is monitoring the situation and considering regulatory action, including a warning label consultation. They will publish their recommendations later this year.
‘There is not enough information at this time to conclusively identify which medicines are higher risk,’ TGA said.
Key Takeaways
- The Therapeutic Goods Administration (TGA) has revealed a link between liver injury and curcumin, an ingredient in turmeric and other spices.
- The risk does not associate with turmeric consumed in typical quantities as a food product but only with medicinal doses of curcumin.
- Over 600 medicines listed in the Australian Register of Therapeutic Goods contain curcumin.
- French Agency for Food, Environmental and Occupational Health & Safety (ANSES) reported adverse effects of curcumin supplements in 2022, citing that the ingredient has poor bioavailability.
- The TGA recommended anyone taking curcumin supplements who experiences possible symptoms of liver damage, such as yellowing skin or eyes, to seek immediate medical advice.
The ANSES and the TGA's warning is an important reminder to stay vigilant when taking supplements. Stay safe, folks!
What do you think of this story, dear members? Do you or anyone you know take turmeric as a supplement? Share your thoughts in the comments!