Patient testimonials reveal the dark side of spinal cord stimulators
By
Seia Ibanez
- Replies 7
The promise of relief from chronic pain is a beacon of hope for many, but recent revelations have cast a shadow over the use of spinal cord stimulators, a medical device designed to alleviate such pain.
The device, which can cost up to $58,000, is implanted near the spinal cord and sends electrical pulses intended to block the sensation of pain.
Marketed as a safe and effective solution, the reality for some patients has been a far cry from the pain-free life they were promised.
Shocking patient testimonials have emerged, painting a grim picture of the complications associated with spinal cord stimulators.
Following a Four Corners investigation, these stories have come to light that exposed significant safety concerns within the multi-billion-dollar chronic pain industry.
One such story is that of Melinda McAdam, who experienced life-threatening complications after her device became infected.
She recalled the experience of being rushed to the hospital and having the stimulator removed.
‘By the time I got to the hospital, I was projectile vomiting green pus,’ she said.
‘I thought I was going to die. I remember when I woke up, a priest was giving me my last rites.’
‘I was informed that the spinal cord stimulator was infected when placed in my spine and that I was septic and…I was very lucky to be alive,’ she added.
44-year-old Navy veteran Lincoln Stewart also shared his distressing experience with the device.
‘My attempt to create a functional life has meant I have had many surgeries and treatments,’ Stewart said.
Despite multiple revisions and reinsertions, Stewart has suffered from infections, sharp leg pains, and sudden falls.
‘I have a split second where an excruciating pain and shock-like feeling goes through my body, and my legs give way. I instantly fall to the ground, and it comes so quickly,’ he said.
The electric shocks he endured are so severe that they have been likened to being zapped by an electric fence.
‘I have woken up to large electric shocks that felt like I was being pulled out of bed by my legs.’
Stewart added that the side effects of the stimulator have outweighed its benefits.
‘I feel like I am in a system of “what's next”,’ he said.
‘I'm always waiting for the next new treatment to reduce my pain.’
Thousands of these devices are sold in Australia each year, and an analysis by Private Healthcare Australia indicated that an estimated 41 per cent require some form of intervention within three years.
The analysis was based on data from six private health insurance funds covering around 6,000 patients with a stimulator for 10 years.
This starkly contrasted with the less than 3 per cent intervention rate for hip replacements over the same period.
The Therapeutic Goods Administration (TGA), Australia's regulator for medical devices, has received over 2,000 reports of adverse events related to spinal cord stimulators since 2012.
These reports include a range of issues, from device malfunction to paralysis, sepsis, burns, electric shocks, and falls.
These alarming statistics have led organisations such as Private Healthcare Australia and the Australian Patients Association (APA) to demand the immediate recall and suspension of spinal cord stimulators, pending the outcome of the TGA's investigation.
‘Australians should be confident they are receiving safe, high-quality health care,’ Private Healthcare CEO Rachel David said.

Health Minister Mark Butler sought an update from the TGA after it launched a review into stimulators, which has been ongoing for more than two years.
The TGA has assured that the review was ‘progressing’ and that they are working as quickly as possible to finalise the post-market review investigations.
‘These devices are complex, and manufacturers are required to hold a substantial amount of evidence, which takes time for the TGA to assess robustly, as each entry is reviewed separately,’ the TGA said.
‘The TGA considers a range of information to make an informed regulatory decision, including expert advice on various matters, including contemporary views on risks and benefits relating to these devices.’
The Medical Technology Association of Australia (MTAA), representing device companies, maintained that spinal cord stimulators have ‘demonstrated the ability to produce clinically meaningful pain relief, improve quality of life and significantly reduce the need for medication usage in individuals suffering with chronic pain.’
However, independent research, including a Cochrane review, has found that these devices may be no better than a placebo procedure and have cited evidence of harm.
Patients like Rebecca Mercer, a mother of two, have had their lives turned upside down by complications from spinal cord stimulator surgeries.
Mercer can no longer work and is in constant pain, stating that the device has 'ruined' her life.
‘I was having these horrendous shocks, which was making me fly up out of bed,’ she said.
The scan showed multiple crush fractures on her spine, and her doctors couldn’t explain how these fractures happened.
A year later, she had the stimulator removed.
‘It didn't work. It stripped me of my quality of life,’ she said.
The TGA said better health and wellbeing for all, including continual assessment of patient safety, was its priority.
‘The TGA strongly encourages any patient concerned with a medical device to report any adverse event or near (potential) adverse event to the TGA, including where an incident involves a healthcare professional.’
You can watch the video below:
Credit: ABC News / YouTube
 What do you think of this story, members? Let us know in the comments below.
What do you think of this story, members? Let us know in the comments below.
The device, which can cost up to $58,000, is implanted near the spinal cord and sends electrical pulses intended to block the sensation of pain.
Marketed as a safe and effective solution, the reality for some patients has been a far cry from the pain-free life they were promised.
Shocking patient testimonials have emerged, painting a grim picture of the complications associated with spinal cord stimulators.
Following a Four Corners investigation, these stories have come to light that exposed significant safety concerns within the multi-billion-dollar chronic pain industry.

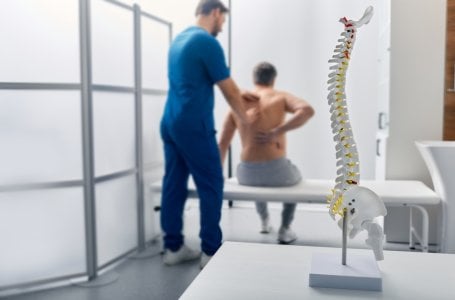
Patients have shared their painful testimonials about the effects of spinal cord stimulators. Credit: Shutterstock
One such story is that of Melinda McAdam, who experienced life-threatening complications after her device became infected.
She recalled the experience of being rushed to the hospital and having the stimulator removed.
‘By the time I got to the hospital, I was projectile vomiting green pus,’ she said.
‘I thought I was going to die. I remember when I woke up, a priest was giving me my last rites.’
‘I was informed that the spinal cord stimulator was infected when placed in my spine and that I was septic and…I was very lucky to be alive,’ she added.
44-year-old Navy veteran Lincoln Stewart also shared his distressing experience with the device.
‘My attempt to create a functional life has meant I have had many surgeries and treatments,’ Stewart said.
Despite multiple revisions and reinsertions, Stewart has suffered from infections, sharp leg pains, and sudden falls.
‘I have a split second where an excruciating pain and shock-like feeling goes through my body, and my legs give way. I instantly fall to the ground, and it comes so quickly,’ he said.
The electric shocks he endured are so severe that they have been likened to being zapped by an electric fence.
‘I have woken up to large electric shocks that felt like I was being pulled out of bed by my legs.’
Stewart added that the side effects of the stimulator have outweighed its benefits.
‘I feel like I am in a system of “what's next”,’ he said.
‘I'm always waiting for the next new treatment to reduce my pain.’
Thousands of these devices are sold in Australia each year, and an analysis by Private Healthcare Australia indicated that an estimated 41 per cent require some form of intervention within three years.
The analysis was based on data from six private health insurance funds covering around 6,000 patients with a stimulator for 10 years.
This starkly contrasted with the less than 3 per cent intervention rate for hip replacements over the same period.
The Therapeutic Goods Administration (TGA), Australia's regulator for medical devices, has received over 2,000 reports of adverse events related to spinal cord stimulators since 2012.
These reports include a range of issues, from device malfunction to paralysis, sepsis, burns, electric shocks, and falls.
These alarming statistics have led organisations such as Private Healthcare Australia and the Australian Patients Association (APA) to demand the immediate recall and suspension of spinal cord stimulators, pending the outcome of the TGA's investigation.
‘Australians should be confident they are receiving safe, high-quality health care,’ Private Healthcare CEO Rachel David said.

Several organisations called for immediate recall and suspension of spinal cord stimulators. Credit: Shutterstock
Health Minister Mark Butler sought an update from the TGA after it launched a review into stimulators, which has been ongoing for more than two years.
The TGA has assured that the review was ‘progressing’ and that they are working as quickly as possible to finalise the post-market review investigations.
‘These devices are complex, and manufacturers are required to hold a substantial amount of evidence, which takes time for the TGA to assess robustly, as each entry is reviewed separately,’ the TGA said.
‘The TGA considers a range of information to make an informed regulatory decision, including expert advice on various matters, including contemporary views on risks and benefits relating to these devices.’
The Medical Technology Association of Australia (MTAA), representing device companies, maintained that spinal cord stimulators have ‘demonstrated the ability to produce clinically meaningful pain relief, improve quality of life and significantly reduce the need for medication usage in individuals suffering with chronic pain.’
However, independent research, including a Cochrane review, has found that these devices may be no better than a placebo procedure and have cited evidence of harm.
Patients like Rebecca Mercer, a mother of two, have had their lives turned upside down by complications from spinal cord stimulator surgeries.
Mercer can no longer work and is in constant pain, stating that the device has 'ruined' her life.
‘I was having these horrendous shocks, which was making me fly up out of bed,’ she said.
The scan showed multiple crush fractures on her spine, and her doctors couldn’t explain how these fractures happened.
A year later, she had the stimulator removed.
‘It didn't work. It stripped me of my quality of life,’ she said.
The TGA said better health and wellbeing for all, including continual assessment of patient safety, was its priority.
‘The TGA strongly encourages any patient concerned with a medical device to report any adverse event or near (potential) adverse event to the TGA, including where an incident involves a healthcare professional.’
You can watch the video below:
Credit: ABC News / YouTube
Key Takeaways
- Horror stories have emerged about patients suffering serious complications from spinal cord stimulators following a Four Corners investigation.
- Despite claims of safety and effectiveness by device companies, independent research indicated spinal cord stimulators may be no better than placebo procedures and can pose dangers.
- The Therapeutic Goods Administration (TGA) is pressured to complete a long-overdue review of spinal cord stimulators after an analysis revealed high patient intervention rates.
- Advocacy groups and some medical professionals are calling for the immediate suspension and recall of spinal cord stimulators, pending the outcome of the TGA's investigation to ensure patient safety.







