Australians are being hurt by this vitamin—here’s how to protect yourself!
By
Maan
- Replies 12
Vitamins are often seen as a harmless way to boost our health—but what happens when something so common carries hidden risks?
A recent recommendation by health authorities has brought one widely used supplement under intense scrutiny.
The findings have sparked debate, concern, and even legal action.
Vitamins have long been marketed as essential allies in our daily health routines—but growing concerns surrounding one common supplement have prompted Australian regulators to take a harder look.
What began as a quiet pattern of side effects has now led to proposed regulatory changes, warnings from the medical community, and the threat of a looming class action lawsuit.
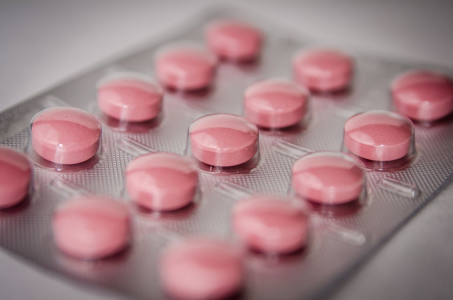
The vitamin in question? B6—also known on ingredient lists as pyridoxine, pyridoxal or pyridoxamine. It's found in everything from energy drinks and weight loss shakes to multivitamins and medicines, appearing in thousands of products sold across Australia.
But according to the Therapeutic Goods Administration (TGA), excessive intake of this seemingly routine supplement has been linked to a range of serious nerve-related conditions—such as peripheral neuropathy and chronic polyneuropathy—with over 170 cases formally reported.
Medical professionals believed that was just the tip of the iceberg.
‘There are likely hundreds of thousands of Australians who are taking far too much vitamin B6,’ said Michael Bonning from the Australian Medical Association.
‘While most of them have no side effects, if you are taking large doses of B6 your risk of peripheral neuropathy goes up.’
‘We know that reported cases of peripheral neuropathy are a massive underestimate of the problem in the community because so many people are unaware that vitamins can cause these symptoms.’
Dr Bonning added that most Australians already get what they need from their diet, echoing the sentiment of a growing chorus of health professionals who’ve been sounding the alarm.
In response to these concerns, a senior TGA medical officer—referred to in documentation as ‘the delegate’—released an Interim Decision outlining several major proposals aimed at tightening regulations and improving public safety.
Central to the recommendations was the reclassification of vitamin B6 supplements containing more than 50mg as Schedule 3 (Pharmacist Only) medicines, with a deadline for implementation set for 1 February 2027. This change would impact roughly 100 products currently available without restriction, requiring pharmacist consultation before purchase.
‘Currently, preparations providing up to 200 mg of [B6]…are available for self-selection without any professional guidance or oversight,’ the report stated.
‘Almost 80 per cent of these products provide a dose of more than 2 mg/day, which is above the RDI for pyridoxine, pyridoxal or pyridoxamine.’
The TGA spokesperson explained that the move aimed to strike a balance between benefits and potential harm.
‘The interim decision balances the risks and benefits of using vitamin B6, including the risk of peripheral neuropathy, acknowledging its potential for irreversible harm at higher doses and variability in individual metabolism,’ they said.
However, the proposed changes only applied to a fraction of the more than 1,500 B6-containing products listed on the Australian Register of Therapeutic Goods. The majority remained unscheduled and freely available over the counter.
The Interim Decision also suggested that products containing more than 200mg of vitamin B6 be reclassified as prescription-only medications under Schedule 4.
Pharmacist Caroline Diamantis, vice-president of the Pharmaceutical Society of Australia, welcomed the idea of stronger pharmacist involvement.
‘It’s a good thing that pharmacists will be able to have a really good counselling session with a patient to make sure the item they’re taking doesn’t interact with other medications,’ she said.
‘People used to think that complimentary medicines were completely safe, but the more we delve into it, the more we understand they do have unwanted side effects or interference with other medications.’
Also under review were product labelling requirements. The Interim Decision called for clearer identification of B6 on supplement packaging and stronger, more direct warning labels.
‘Use of ingredient names in labelling is inconsistent and confusing for consumers … and not always described as vitamin B6,’ the report said.
One proposed warning read: ‘Excess consumption of vitamin B6 can cause nerve damage. Stop use and see a doctor if you experience tingling, burning or numbness.’
Of particular concern were products that made no mention of B6 on the front of the label, leaving consumers in the dark about their intake levels.
The report argued that current labelling placed too much pressure on consumers to calculate daily dosage themselves—something beyond the health literacy of many.
The changes triggered mixed reactions across the complementary medicines industry.
John O’Doherty, CEO of Complementary Medicines Australia, acknowledged the report but questioned the alarm.
‘We’ll be carefully reviewing the interim decision and the recommendations of the Advisory Committee on Medicines Scheduling,’ he said.
He claimed adverse reactions were ‘extremely rare’, saying: ‘Based on a comparison of sales data with the TGA’s public Database of Adverse Events, the chance of a reaction is less than one in half a million — this is extremely rare.’
But prior investigations by 7.30 found dozens of Australians who had experienced B6 toxicity—many of whom only connected their symptoms to the vitamin after watching the program’s coverage.
The TGA itself remained cautious.
‘The true rate of occurrence of an adverse event cannot be determined from spontaneous adverse event reporting systems due to both general under-reporting and a lack of usage data,’ the delegate wrote.
‘A low number of spontaneous adverse event reports cannot be considered evidence of the absence of a safety issue.’
Mr O’Doherty did, however, back clearer labelling, noting his organisation had been pushing for simplified language.
‘At CMA’s request, this is now under internal review at the TGA,’ he said.
Meanwhile, legal action appeared to be gaining traction.
Medical negligence lawyer Nick Mann, from the firm Polaris, said the Interim Decision was a welcome first step—but one that didn’t go far enough. His firm launched a class action against Blackmores, one of the largest players in the complementary medicines space.
‘We continue to investigate the potential class action, and given the response, the support and the evidence that’s been pouring in…with over 140 people contacting us to date,’ he said.
‘Each of them had heartbreaking stories about how their lives have been affected by B6 toxicity, and sadly, many of them continue to be affected long after ceasing supplements containing Vitamin B6.’
‘We’ve also had at least 10 doctors contact us to tell us about their own experiences with B6, and to let us know that they have discovered B6 toxicity in several of their patients, after taking supplements containing higher than the RDI of the vitamin over weeks and months.’
With the proposed deadline still over a year away, the conversation around vitamin B6 and supplement safety is far from over.
Want to dive deeper into what makes this vitamin so risky? Watch this quick explainer to find out what to look for—and how to stay one step ahead.
Source: Youtube/doctorjanine

With so many Aussies taking daily supplements without a second thought, do you double-check what’s really in yours? Let us know your thoughts in the comments.
In a previous story, we looked into a surprising health risk hiding in one of Australia's favourite drinks—proof that even everyday items can come with unexpected dangers.
For older Aussies trying to stay on top of their health, these hidden risks in common products are a real concern.
If you're keeping an eye on what goes into your body, this one’s worth a read too.
Read more: Favourite drink puts you at risk as massive recall reveals hidden safety concern
A recent recommendation by health authorities has brought one widely used supplement under intense scrutiny.
The findings have sparked debate, concern, and even legal action.
Vitamins have long been marketed as essential allies in our daily health routines—but growing concerns surrounding one common supplement have prompted Australian regulators to take a harder look.
What began as a quiet pattern of side effects has now led to proposed regulatory changes, warnings from the medical community, and the threat of a looming class action lawsuit.
The vitamin in question? B6—also known on ingredient lists as pyridoxine, pyridoxal or pyridoxamine. It's found in everything from energy drinks and weight loss shakes to multivitamins and medicines, appearing in thousands of products sold across Australia.
But according to the Therapeutic Goods Administration (TGA), excessive intake of this seemingly routine supplement has been linked to a range of serious nerve-related conditions—such as peripheral neuropathy and chronic polyneuropathy—with over 170 cases formally reported.
Medical professionals believed that was just the tip of the iceberg.
‘There are likely hundreds of thousands of Australians who are taking far too much vitamin B6,’ said Michael Bonning from the Australian Medical Association.
‘While most of them have no side effects, if you are taking large doses of B6 your risk of peripheral neuropathy goes up.’
‘We know that reported cases of peripheral neuropathy are a massive underestimate of the problem in the community because so many people are unaware that vitamins can cause these symptoms.’
Dr Bonning added that most Australians already get what they need from their diet, echoing the sentiment of a growing chorus of health professionals who’ve been sounding the alarm.
In response to these concerns, a senior TGA medical officer—referred to in documentation as ‘the delegate’—released an Interim Decision outlining several major proposals aimed at tightening regulations and improving public safety.
Central to the recommendations was the reclassification of vitamin B6 supplements containing more than 50mg as Schedule 3 (Pharmacist Only) medicines, with a deadline for implementation set for 1 February 2027. This change would impact roughly 100 products currently available without restriction, requiring pharmacist consultation before purchase.
‘Currently, preparations providing up to 200 mg of [B6]…are available for self-selection without any professional guidance or oversight,’ the report stated.
‘Almost 80 per cent of these products provide a dose of more than 2 mg/day, which is above the RDI for pyridoxine, pyridoxal or pyridoxamine.’
The TGA spokesperson explained that the move aimed to strike a balance between benefits and potential harm.
‘The interim decision balances the risks and benefits of using vitamin B6, including the risk of peripheral neuropathy, acknowledging its potential for irreversible harm at higher doses and variability in individual metabolism,’ they said.
However, the proposed changes only applied to a fraction of the more than 1,500 B6-containing products listed on the Australian Register of Therapeutic Goods. The majority remained unscheduled and freely available over the counter.
The Interim Decision also suggested that products containing more than 200mg of vitamin B6 be reclassified as prescription-only medications under Schedule 4.
Pharmacist Caroline Diamantis, vice-president of the Pharmaceutical Society of Australia, welcomed the idea of stronger pharmacist involvement.
‘It’s a good thing that pharmacists will be able to have a really good counselling session with a patient to make sure the item they’re taking doesn’t interact with other medications,’ she said.
‘People used to think that complimentary medicines were completely safe, but the more we delve into it, the more we understand they do have unwanted side effects or interference with other medications.’
Also under review were product labelling requirements. The Interim Decision called for clearer identification of B6 on supplement packaging and stronger, more direct warning labels.
‘Use of ingredient names in labelling is inconsistent and confusing for consumers … and not always described as vitamin B6,’ the report said.
One proposed warning read: ‘Excess consumption of vitamin B6 can cause nerve damage. Stop use and see a doctor if you experience tingling, burning or numbness.’
Of particular concern were products that made no mention of B6 on the front of the label, leaving consumers in the dark about their intake levels.
The report argued that current labelling placed too much pressure on consumers to calculate daily dosage themselves—something beyond the health literacy of many.
The changes triggered mixed reactions across the complementary medicines industry.
John O’Doherty, CEO of Complementary Medicines Australia, acknowledged the report but questioned the alarm.
‘We’ll be carefully reviewing the interim decision and the recommendations of the Advisory Committee on Medicines Scheduling,’ he said.
He claimed adverse reactions were ‘extremely rare’, saying: ‘Based on a comparison of sales data with the TGA’s public Database of Adverse Events, the chance of a reaction is less than one in half a million — this is extremely rare.’
But prior investigations by 7.30 found dozens of Australians who had experienced B6 toxicity—many of whom only connected their symptoms to the vitamin after watching the program’s coverage.
The TGA itself remained cautious.
‘The true rate of occurrence of an adverse event cannot be determined from spontaneous adverse event reporting systems due to both general under-reporting and a lack of usage data,’ the delegate wrote.
‘A low number of spontaneous adverse event reports cannot be considered evidence of the absence of a safety issue.’
Mr O’Doherty did, however, back clearer labelling, noting his organisation had been pushing for simplified language.
‘At CMA’s request, this is now under internal review at the TGA,’ he said.
Meanwhile, legal action appeared to be gaining traction.
Medical negligence lawyer Nick Mann, from the firm Polaris, said the Interim Decision was a welcome first step—but one that didn’t go far enough. His firm launched a class action against Blackmores, one of the largest players in the complementary medicines space.
‘We continue to investigate the potential class action, and given the response, the support and the evidence that’s been pouring in…with over 140 people contacting us to date,’ he said.
‘Each of them had heartbreaking stories about how their lives have been affected by B6 toxicity, and sadly, many of them continue to be affected long after ceasing supplements containing Vitamin B6.’
‘We’ve also had at least 10 doctors contact us to tell us about their own experiences with B6, and to let us know that they have discovered B6 toxicity in several of their patients, after taking supplements containing higher than the RDI of the vitamin over weeks and months.’
With the proposed deadline still over a year away, the conversation around vitamin B6 and supplement safety is far from over.
Want to dive deeper into what makes this vitamin so risky? Watch this quick explainer to find out what to look for—and how to stay one step ahead.
Source: Youtube/doctorjanine
Key Takeaways
- Australian regulators warned that excessive vitamin B6 intake has been linked to serious nerve damage, with over 170 reported cases.
- New rules may reclassify high-dose B6 products as pharmacist-only or prescription-only medicines by 1 February 2027.
- Experts called for clearer labelling, as many supplements don’t clearly state they contain B6 or how much.
- Legal action is underway against Blackmores, with over 140 people claiming long-term harm from B6 toxicity.
With so many Aussies taking daily supplements without a second thought, do you double-check what’s really in yours? Let us know your thoughts in the comments.
In a previous story, we looked into a surprising health risk hiding in one of Australia's favourite drinks—proof that even everyday items can come with unexpected dangers.
For older Aussies trying to stay on top of their health, these hidden risks in common products are a real concern.
If you're keeping an eye on what goes into your body, this one’s worth a read too.
Read more: Favourite drink puts you at risk as massive recall reveals hidden safety concern